What is Corrosion ?
CORROSION SCIENCE
Toddy we learn about the corrosion
science that is what is corrosion, how to corrosion process is occurring and
how to prevent it. Let’s start learning, first of all we learn about what is
corrosion.
Definition of
Corrosion:
Corrosion is the presses of destruction
or decomposition of metal by electrochemical and chemical attack of environment
on the surface of metals called corrosion. The machines, equipment, various
metallic structure and metallic parts damage due to corrosion. The corrosion of
metals by a chemical attach due to environment is slow but rate of corrosion is
fast due to formation of cell. During the formation of cell there are two types
of cell is formed.
1)
Electrochemical Cell
2)
Electrolyte Cell
Types of Corrosion:
Direct Chemical Corrosion:
The corrosion occurring due to direct attack of chemicals like atmospheric gases, hydrogen, oxygen, carbon dioxide, chlorine, nitrogen oxide etc and attack of hot flow liquids on the metals. The rate of this corrosion is depending upon the following factors.
The corrosion occurring due to direct attack of chemicals like atmospheric gases, hydrogen, oxygen, carbon dioxide, chlorine, nitrogen oxide etc and attack of hot flow liquids on the metals. The rate of this corrosion is depending upon the following factors.
- Temperature
- Moisture in Air
- Nature of Oxide Film on Surface of Metal
- Chemical Affinity between Metal and Gas
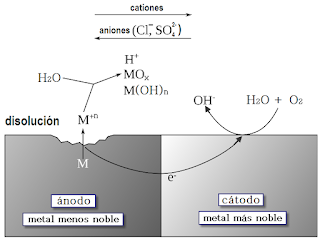
Electrochemical or Wet
Corrosion:
The corrosion of metal by the aqueous
conduction medium with the formation of catholic and anodic areas is called
electrochemical corrosion. The aqueous medium may be aqueous solution, see,
water, moisture, and high humidity. In this corrosion anodic and catholic
area are form but corrosion occur only
anodic aria and corrosion product is desolve into medium or deposited into
surface of cathode.
This types of corrosion have two types.
1) Galvanic
Corrosion
2)
Concentration Cell Corrosion
1) Galvani Corrosion:
Galvanic
corrosion is electrochemical corrosion of a metal by attack of conducting
medium, with the formation of galvanic cell is called galvanic corrosion.
2) Concentration Cell Corrosion:
When a part of metal is contact
with higher concentration of electrolyte and other part of metal is formed
concentration cell .The part of metal which is contact with the low
concentration of electrolyte act as anode and get corroded, this corrosion is
called concentration cell corrosion. In easy word we say that "The
concentration cell formed due to warring of Oxygen concentration on a metal,
are called Concentration Cell Corrosion".









This comment has been removed by a blog administrator.
ReplyDelete